Abstract
Introduction:
Cytomegalovirus (CMV) infection is a common complication after allogeneic hematopoietic-cell transplantation (alloHCT) causing significant morbidity and mortality. The standard approach for preventing clinically significant CMV infection (CS-CMVi) and CMV disease (CMVd), is preemptive therapy (PET) upon detection of CMV reactivation. In 2017 letermovir was approved for CMV prophylaxis in CMV-seropositive allogeneic HCT recipients from transplantation through day 100 after alloHCT. At 2020, letermovir was approved in Israel for prophylaxis in CMV-seropositive alloHCT recipients who were at higher risk for CMV reactivation (seronegative donor, HLA mismatched unrelated donor, haploidentical family donor, ex-vivo T cell depleted graft or recipient suffering from acute Graft Versus Host Disease (aGVHD) requiring >1mg/kg steroid therapy). There is paucity of real-world data regarding the efficacy and outcomes of letermovir prophylaxis in the post-transplant setting. The aim of our study was to report the real-world Israeli experience with letermovir prophylaxis in terms of incidence of CS-CMVi, CMVd and aGVHD, chronic Graft Versus Host Disease (cGVHD), survival and all-cause mortality.
Methods:
We retrospectively analyzed a cohort of 45 alloHCT recipients at high risk for CMV reactivation, from 4 Israeli transplant centers, who underwent alloHCT between January 2020 and -December 2021 and received letermovir prophylaxis (study group). A comparison was performed, to a matched historical cohort of 45 alloHCT recipients, who underwent alloHCT between 2014 and 2020 and were treated according to PET policy (control group). Matching was guided by the risk for CMV reactivation.
Results:
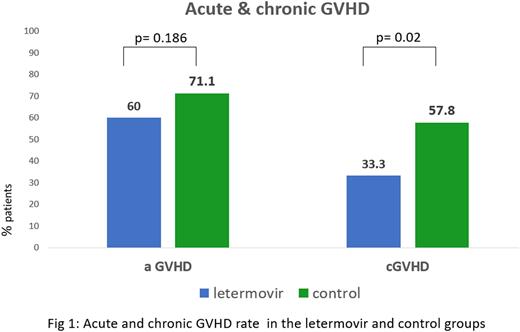
Demographics (recipient's age, gender, ethnicity), disease and pre-transplant characteristics (disease status at transplantation, donor match, conditioning intensity, graft source) of the two groups were comparable accept for a higher percentage of ABO minor mismatch in the control group versus the study group (37% compared to 6%, respectively; p=0.028). There was a higher rate of CS-CMVi during the first 100 days post-transplant in the control group versus the study group (53/3% compared to 35.6%, respectively; p=0.09). There was no difference in CMVd rate between the two groups as well as in relapse rate and overall survival. Acute and chronic GVHD incidence was higher in the control group compared to the study group (71.1% vs 60%; p=0.186 and 57.8% vs 33.3%; p= 0.02, respectively).
Conclusions:
Our real -world Israeli experience cohort of letermovir prophylaxis in high risk alloHCT recipients demonstrates a significant reduction in GVHD occurrence in patients receiving letermovir prophylaxis. GVHD is a significant cause of morbidity and mortality in alloHCT recipients. Our real-world experience is in line with the well-established contiguity and causal relation between CMV infection and GVHD, showing a reduction in CS-CMVi during the prophylaxis period with a corresponding reduction in acute and chronic GVHD. Our cohort is limited by its retrospective nature and a relatively small sample size.
Disclosures
Ram:Takeda: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau. Yehudai-Ofir:Novartis: Other: Advisory board 2021; Gilead: Other: Advisory board 2021. Zuckerman:Orgenesis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BioSight Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cellect Biotechnology: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau. Avni:MSD: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal